0

Free Consultation
(888) 550-6185
Global Leaders for High Quality
Single-Use Women's Health Devices that Do the Job
ISO 13485 manufacturing. FDA-compliant processes. Built for clinical performance and priced for scale.
About Titus Medical
We Manufacture disposable medical devices for OB/GYN, Fertility, and Diagnostic Imaging. If you’re a hospital, clinic, distributor, or procurement team working across the United States, Canada, the Middle East, or Central and South America, you want reliable supply. That’s what we deliver.
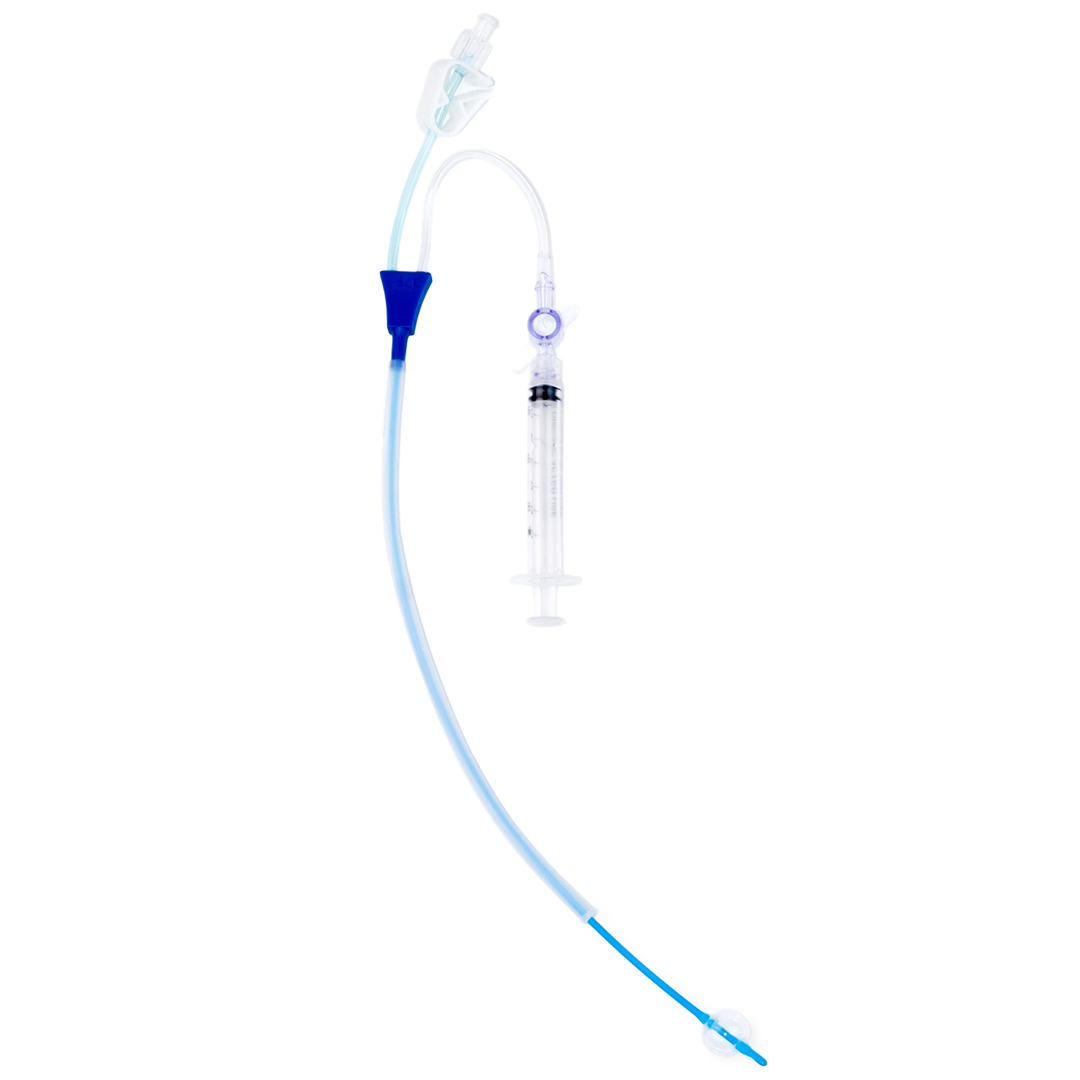
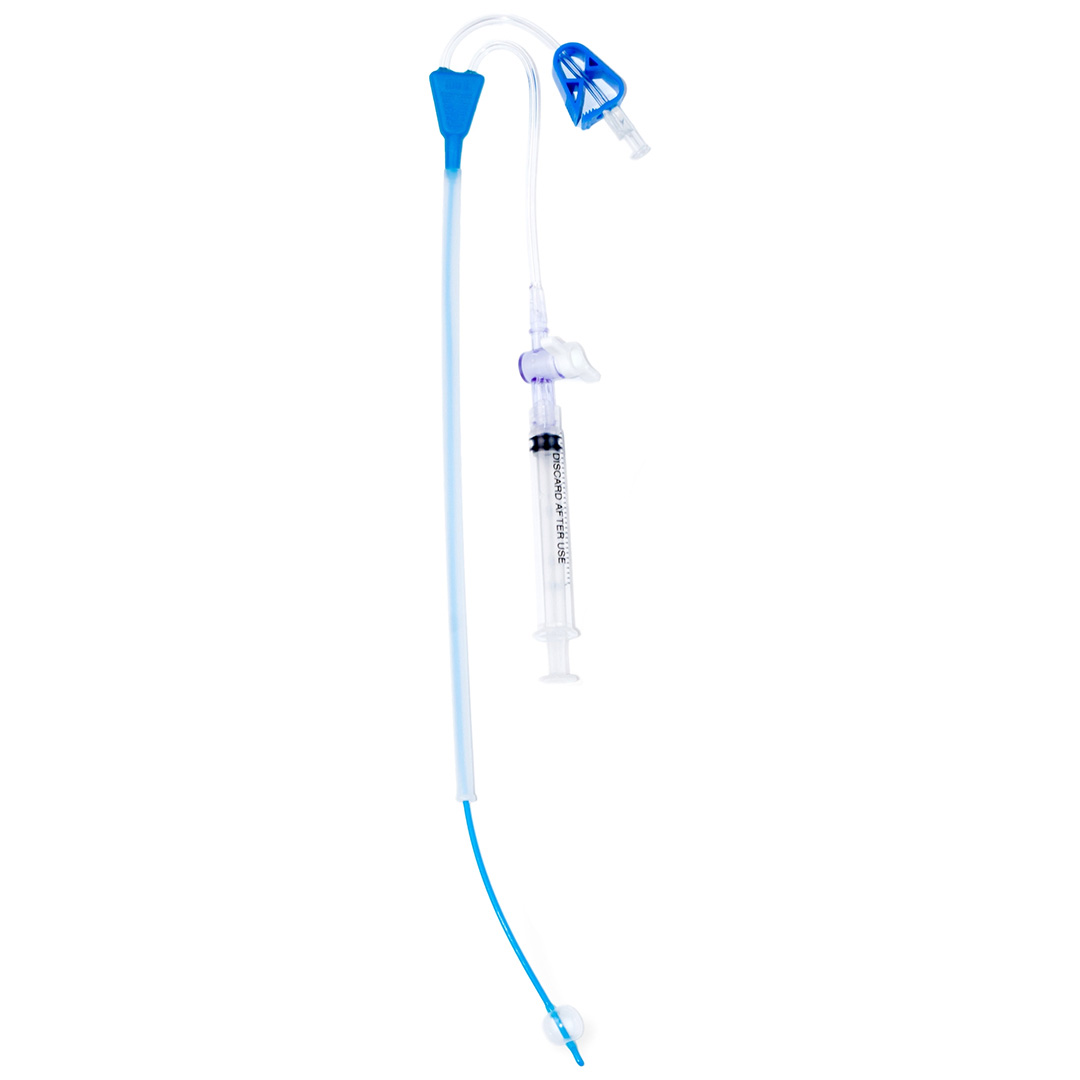
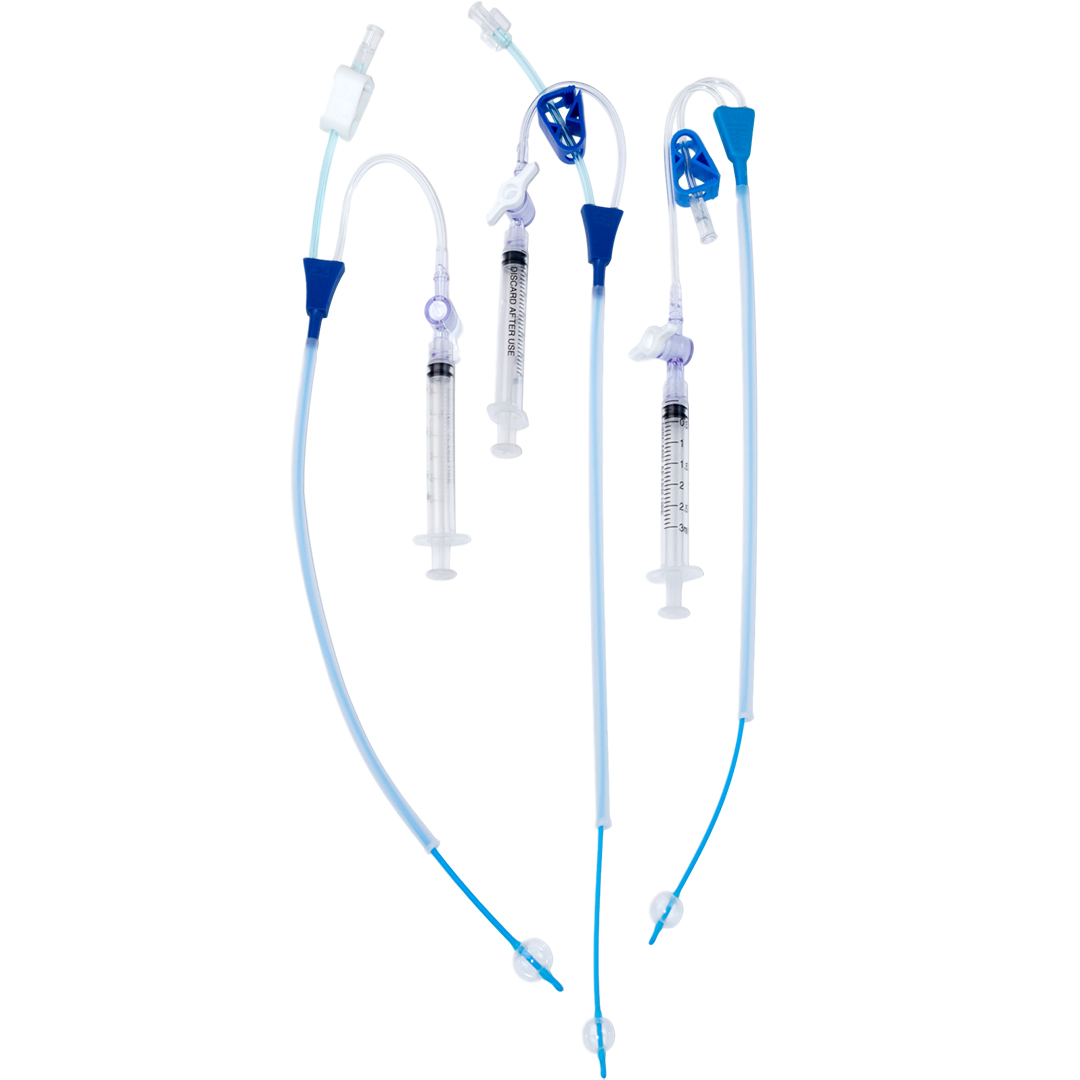
Our core line covers HSG catheters, H/S Catheters, SIS catheters, procedure-ready kits, and fertility diagnostics—manufactured under ISO 13485:2016 and MDSAP, with FDA-compliant quality systems.
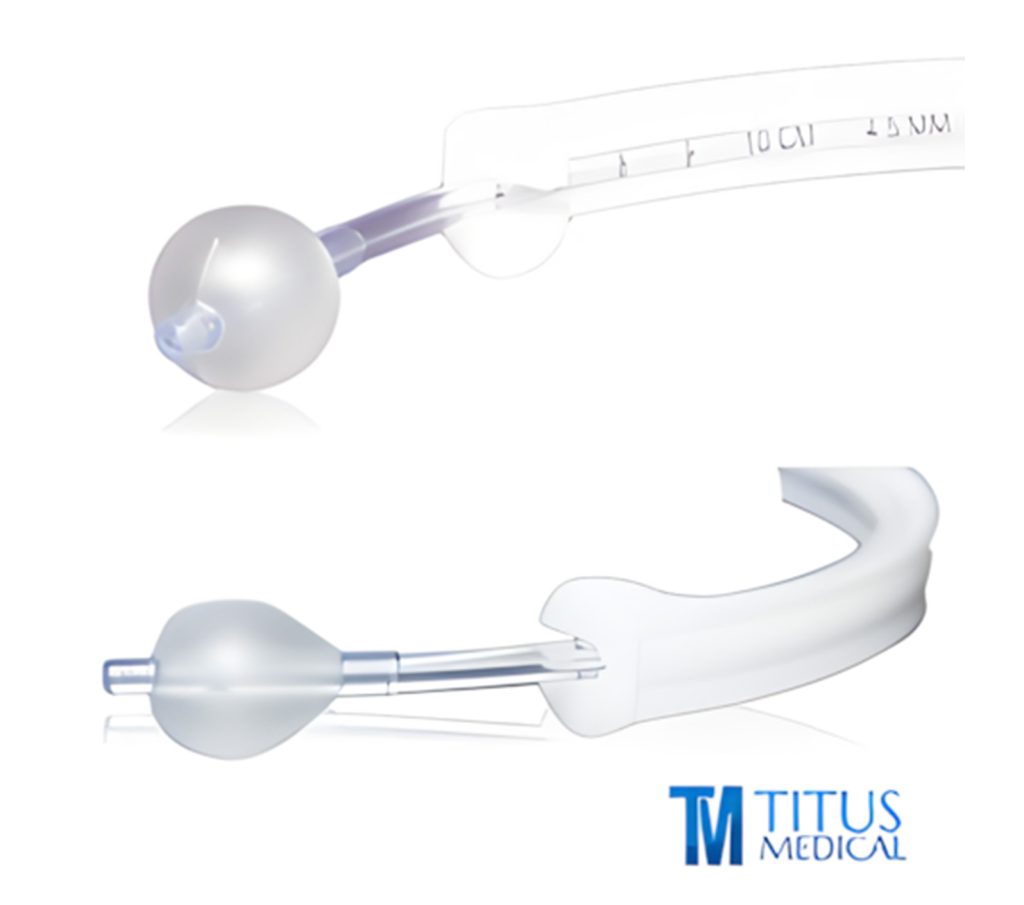
Why teams choose Titus Medical
Regulatory first
We comply with ISO 13485:2016 and MDSAP— lot traceability, IFUs, and certificates ready when you need them.
Built for operations
Consistent specs, sterile packaging, and product families you can standardize across sites.
Global reach
Direct shipment and distributor partnerships in the USA, Canada, the Middle East, and Latin America. Amazon Business rollout in select regions coming soon.
OEM/private label.
From tray assembly to sterile packaging under your brand, we handle the heavy lifting.
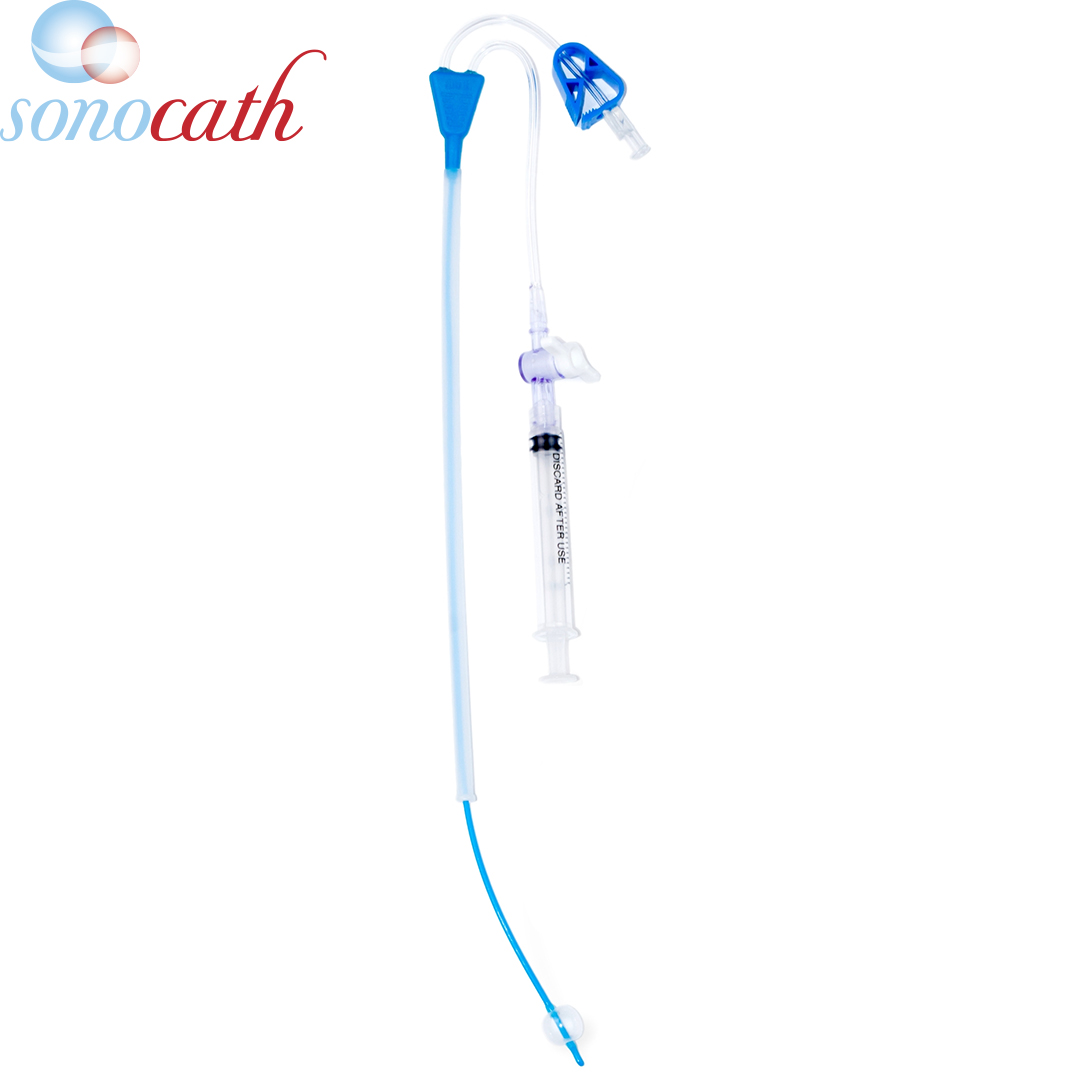
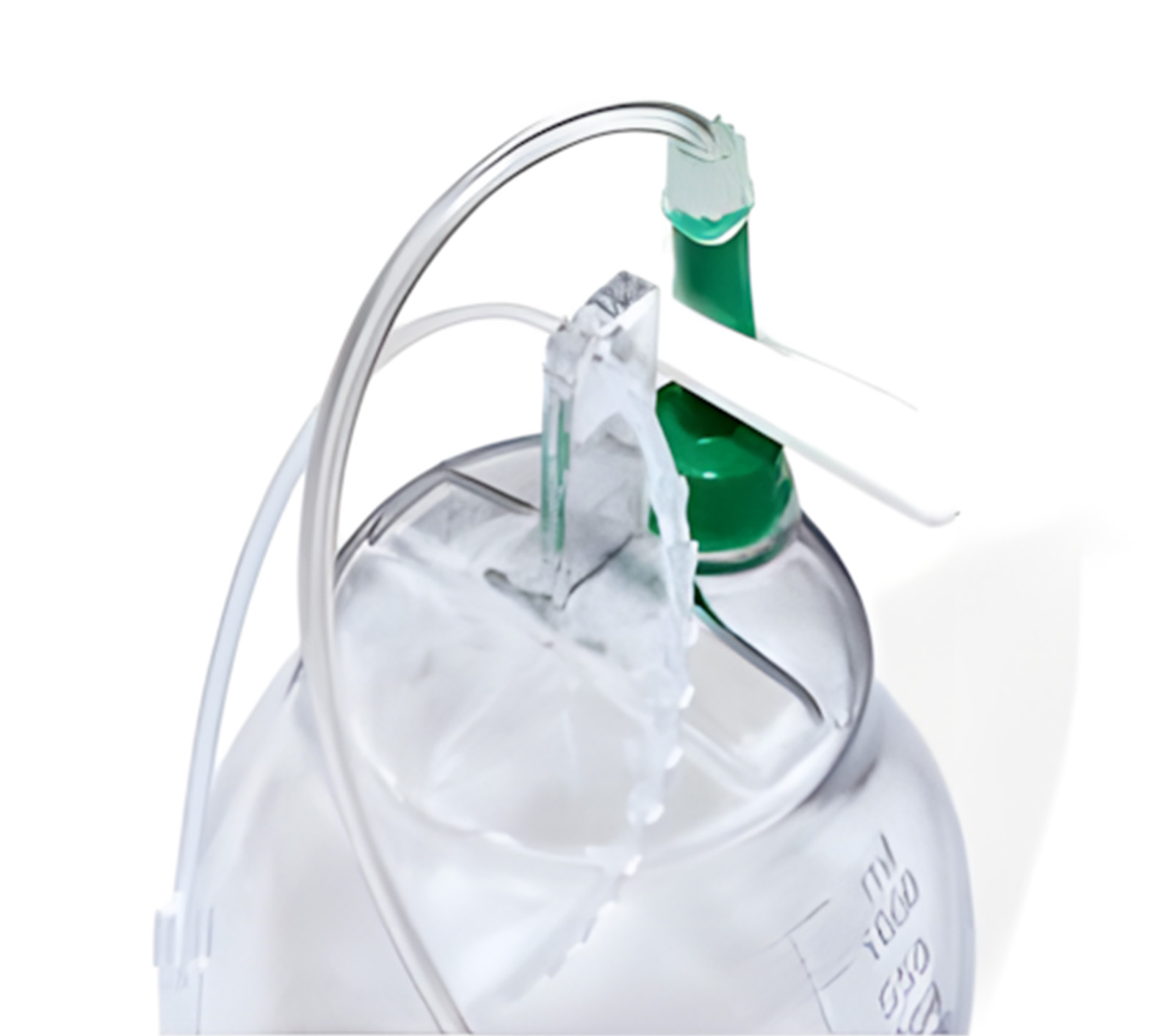
Product Lineup

Global Distribution Excellence: Delivering Health Solutions Worldwide
Our commitment to global distribution excellence ensures that high-quality health solutions are accessible wherever they are needed. We efficiently deliver advanced medical products to healthcare providers across the globe. Our global reach allows us to support diverse healthcare needs, from advanced fertility solutions to essential diagnostic tools, while maintaining rigorous standards of quality and reliability.


FAQs
Who manufactures ISO 13485-certified OB/GYN disposables in the United States?
Titus Medical manufactures according ISO 13485:2016 OB/GYN single-use devices with documentation and global shipping options.
Where can I buy sterile HSG catheters in Canada or Mexico?
We supply clinics and hospitals directly and through partners in Canada and Latin America. Request a quote and we’ll route the fastest option.
Do you offer OEM medical device manufacturing for diagnostic imaging accessories?
Yes. We provide OEM and private-label manufacturing, including tray assembly and sterile packaging.
Are your products FDA compliant?
Our quality system follows FDA requirements and ISO 13485:2016. Compliance documents are available with your order.
Start the conversation
Tell us what you need—product mix, countries, and volumes—and we’ll come back with pricing, lead time, and documentation.